Algae Media
Fertilizer for algae:
Just like your plants at home use fertilizer, so does algae. The nutrients are the same, but often in different proportions. Mainly, nutrients are composed of:
N:P:K and trace elements
- N-Nitrogen
- P-Phosphorus
- K-Potassium
These nutrients can exist in a solid, liquid, or semi-solid medium called a growth medium. Either phase can be used to support the growth of micro-algae cells but each type of media are used for different growing purposes.
Salt broth and agar plate are the two major types of growth media used for cell culture. Typically, cultures are grown in a liquid salt broth with corresponding nutrients and maintained under optimal conditions. However, if you wish to maintain cultures for a longer period of time with less maintenance between transfers, or want to make back-up stock cultures in case things go awry with your liquid cultures, it can be beneficial to make agar plates using your media recipe of choice.
Our Spirulina Nutrient Solution Preparation
Below are instructions on how to make the spirulina media, or you can simply buy it from our store! Here is our link.
First prepare the necessary stock solutions. Next, prepare Solutions I and II; Solution II includes 1 mL of the trace metals stock solution but not the vitamin stock. Autoclave Solutions I and II separately and cool; aseptically combine the two solutions. Aseptically add 1 mL of the cyanocobalamin (B12) solution.
Table 1: Chemical Composition
| Components | Stock Solution | Quantity | Molar Concentration in Final Medium |
| Solution I | 500 mL | ---- | --- |
| NaHCO3 | --- | 13.61 g | 1.62 x 10-4 M |
| Na2CO3 | ---- | 4.03 g | 3.80 x 10-5 M |
| K2HPO4 | --- | 0.50 g | 2.87 x 10-6 M |
| Solution II | 500 mL | --- | -- |
| NaNO3 | -- | 2.50 g | 2.94 x10-5 M |
| K2SO4 | --- | 1.0 g | 5.74 x 10-6 M |
| NaCl | --- | 1.0 g | 1.71 x10-5 M |
| MgSO4 7(H2O) | --- | 0.20 g | 8.11 x10-7 M |
| CaCl2 2(H2O) | -- | 0.04 g | 2.72 x 10-7 M |
| FeSO4 | --- | 0.01 g | 3.60 x 10-8 M |
| Na2EDTA | --- | 0.08 g | 2.15 x10-7 M |
| Trace Metals Solution | (See table 2) | 1.0 mL | ---- |
| Vitamin Stock Solution | (See table 3) | 1 mL | --- |
Trace Metals Solutions
Begin with 900 mL of dH2O and dissolve the EDTA. Independently, dissolve each component and bring final volume to 1 liter. Schlösser (1994) recommends making two trace metals solutions, (a) 0.4 g EDTA and 0.7 g FeSO4 7H2O in 100 mL dH2O, and (b) 0.4 g EDTA and remaining elements in 900 mL dH2O; autoclave separately and combine aseptically when cool.
| Components | Primary Stock Solution | Quantity | Molar Concentration in Final Medium |
| Na2(EDTA)2(H2O) | --- | 0.8 g | 2.15 x 10-6 M |
| FeSO4 7(H2O) | --- | 0.7 g | 2.52 x10-6 M |
| ZnSO4 7(H2O) | 1.0 g L-1 dH2O | 1 mL | 3.48 x 10-9 M |
| MnSO4 7(H2O) | 2.0 g L-1 dH2O | 1 mL | 8.97 x10-9 M |
| H3BO3 | 10.0 g L-1 dH2O | 1 mL |
1.62 x10-7 M |
| Co(NO3)2 6(H2O) | 1.0 g L-1 dH2O | 1 mL |
3.44 x10-9 M |
| Na2MoO4 2(H2O) | 1.0 g L-1 dH2O | 1 mL |
4.13 x10-9 M |
| CuSO4 5(H2O) | 0.005 g L-1 dH2O | 1 mL |
2.00 x10-11 M |
Cyanocobalimin Stock Solution
Dissolve the cyanocobalamin in 1 liter of dH2O and filter sterilize. Store frozen.
Table 3: Vitamin Composition
| Components | Primary Stock Solution | Quantity | Molar Concentration in Final Medium |
| Cyanocobalomin | --- | 0.5 mg | 3.69 x 10-9 M |
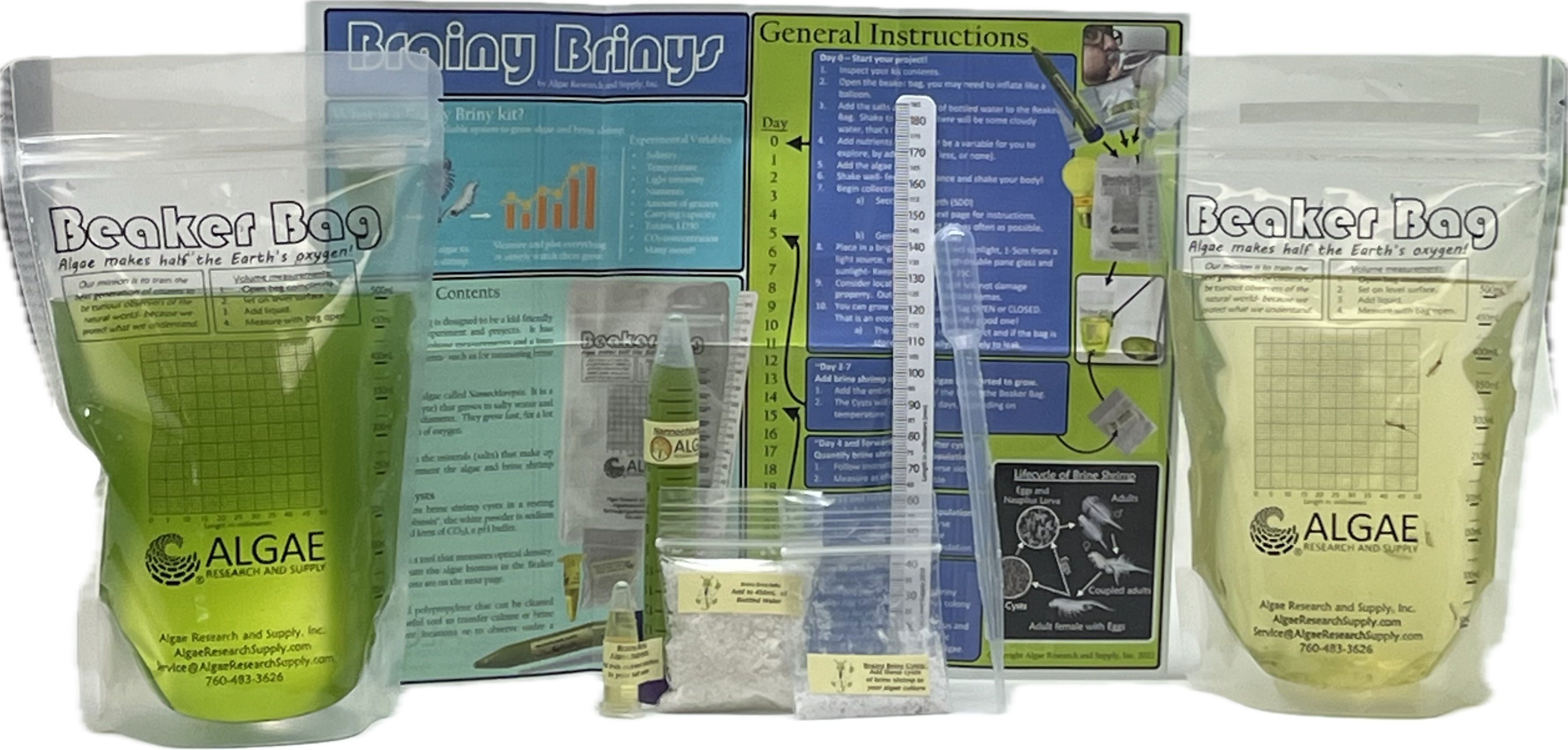
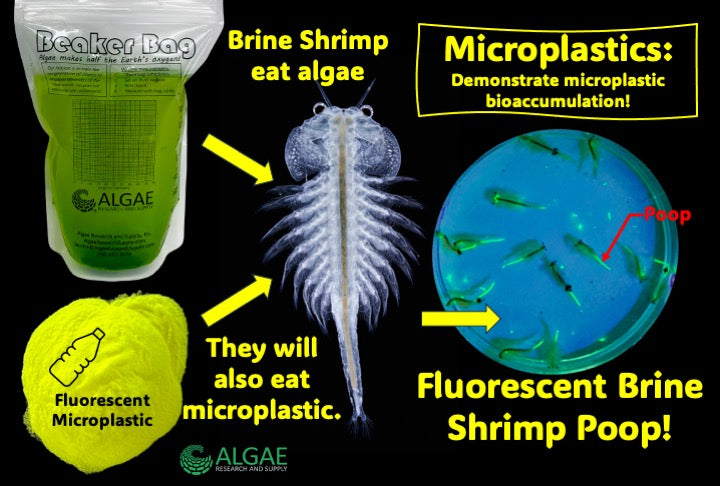
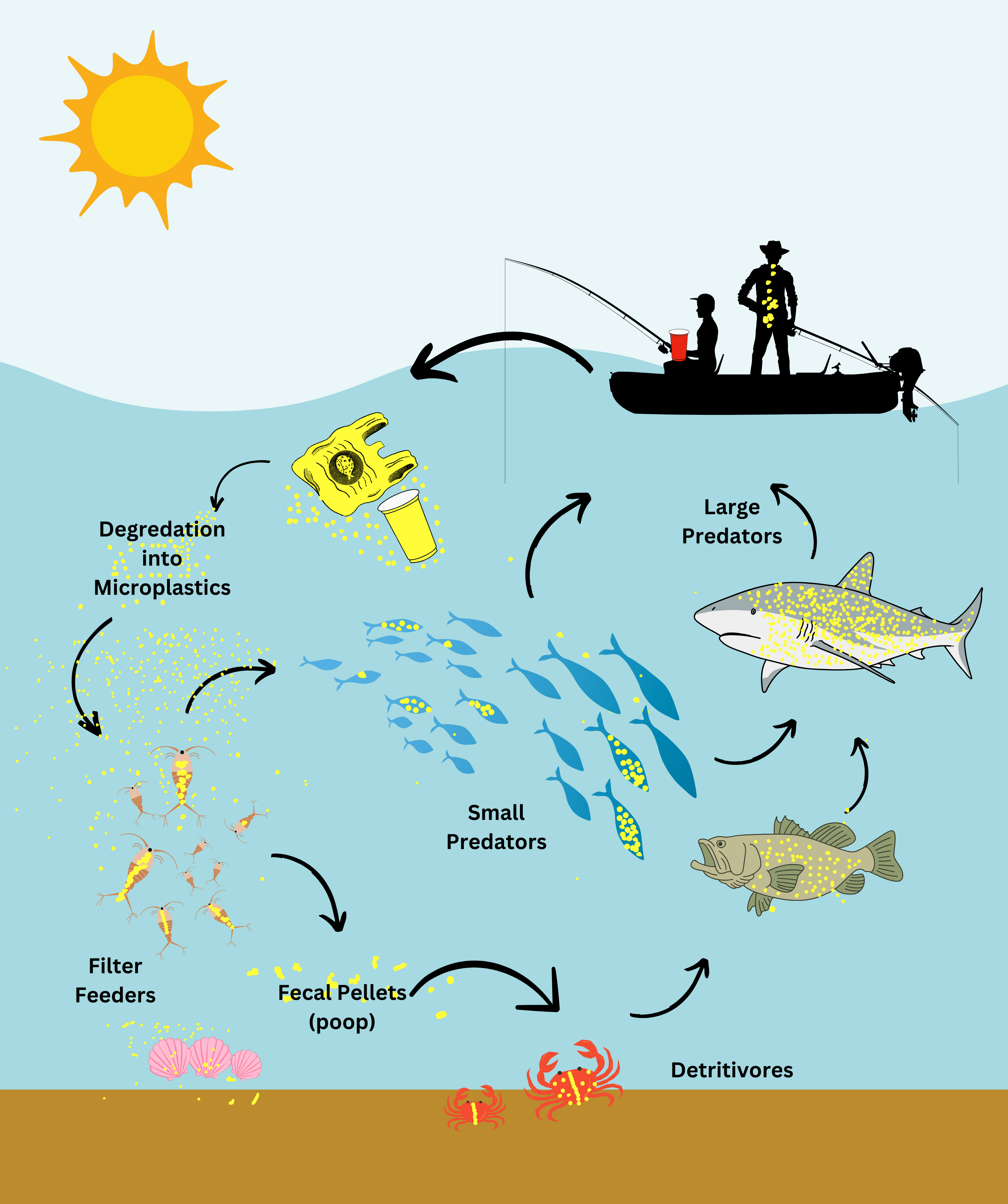
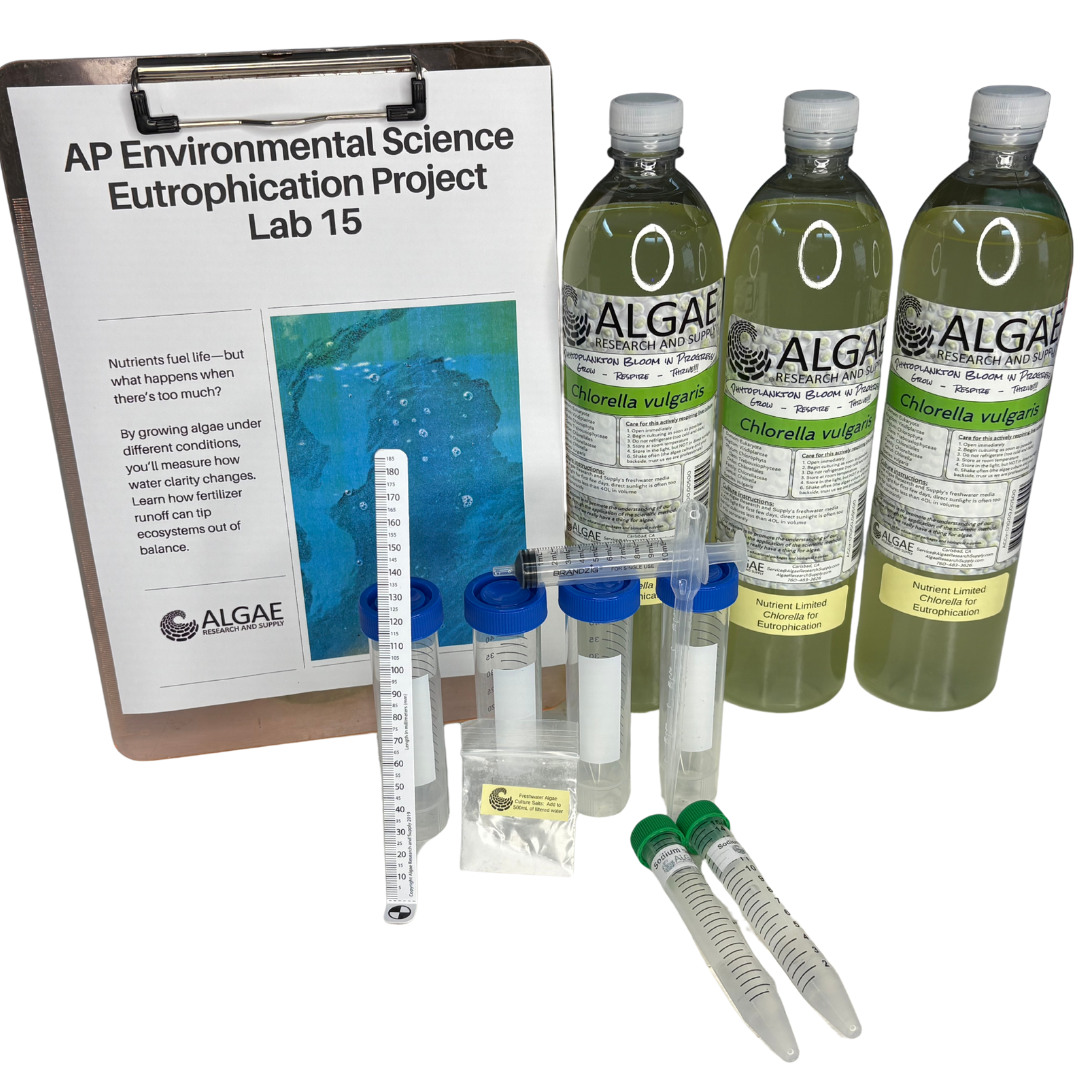
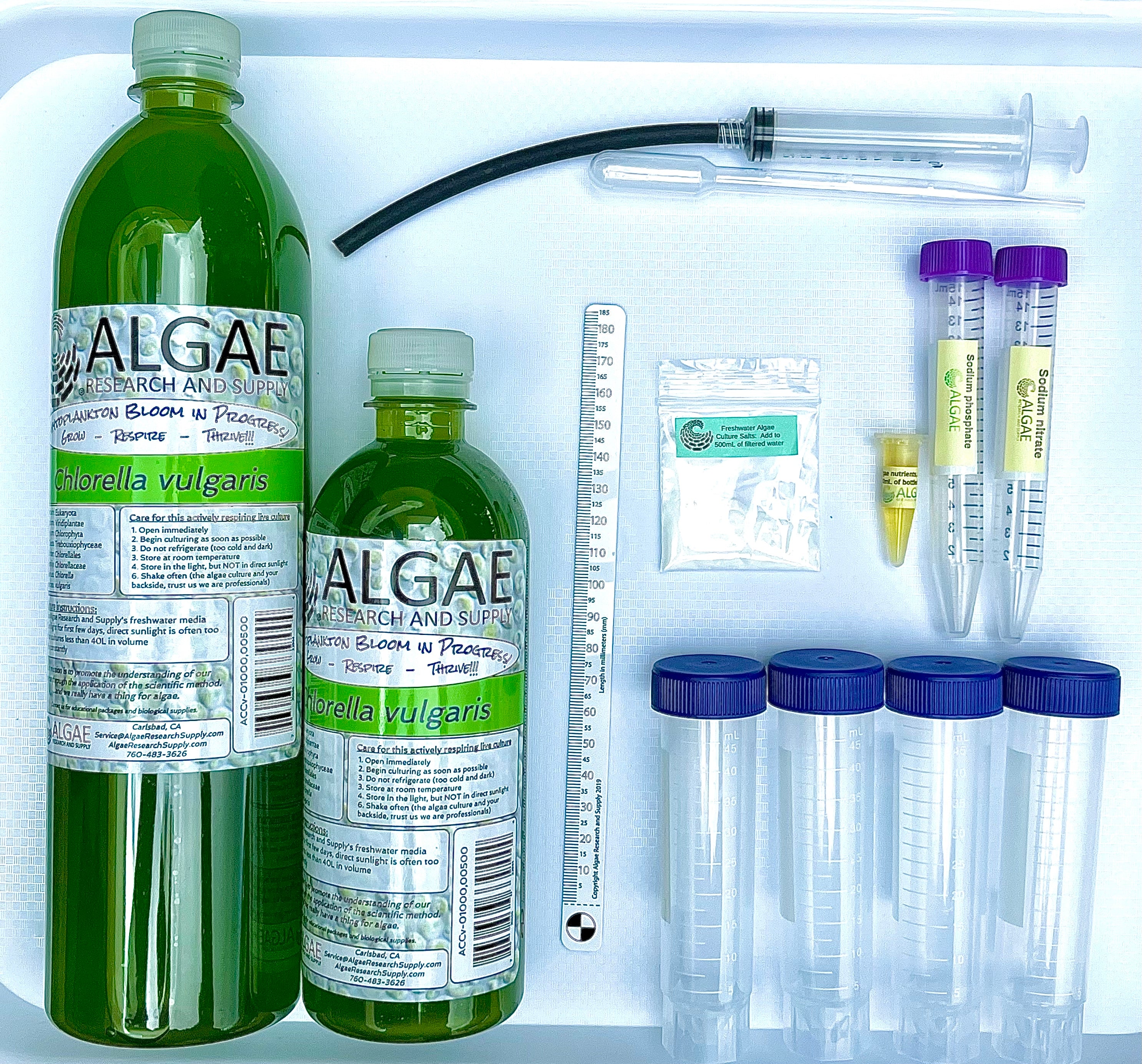
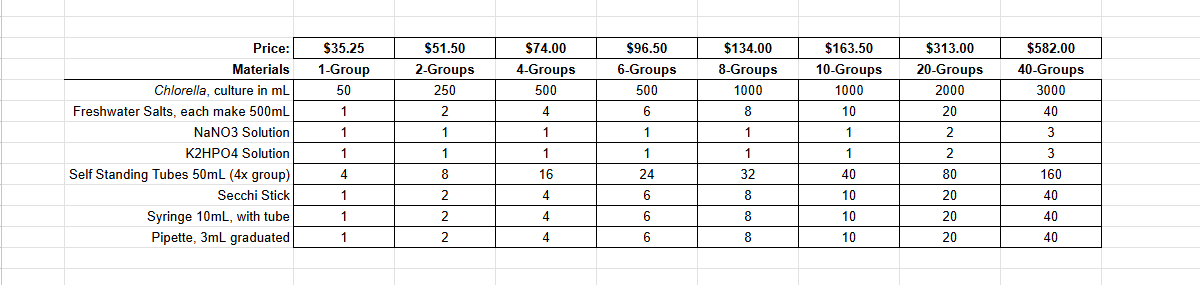
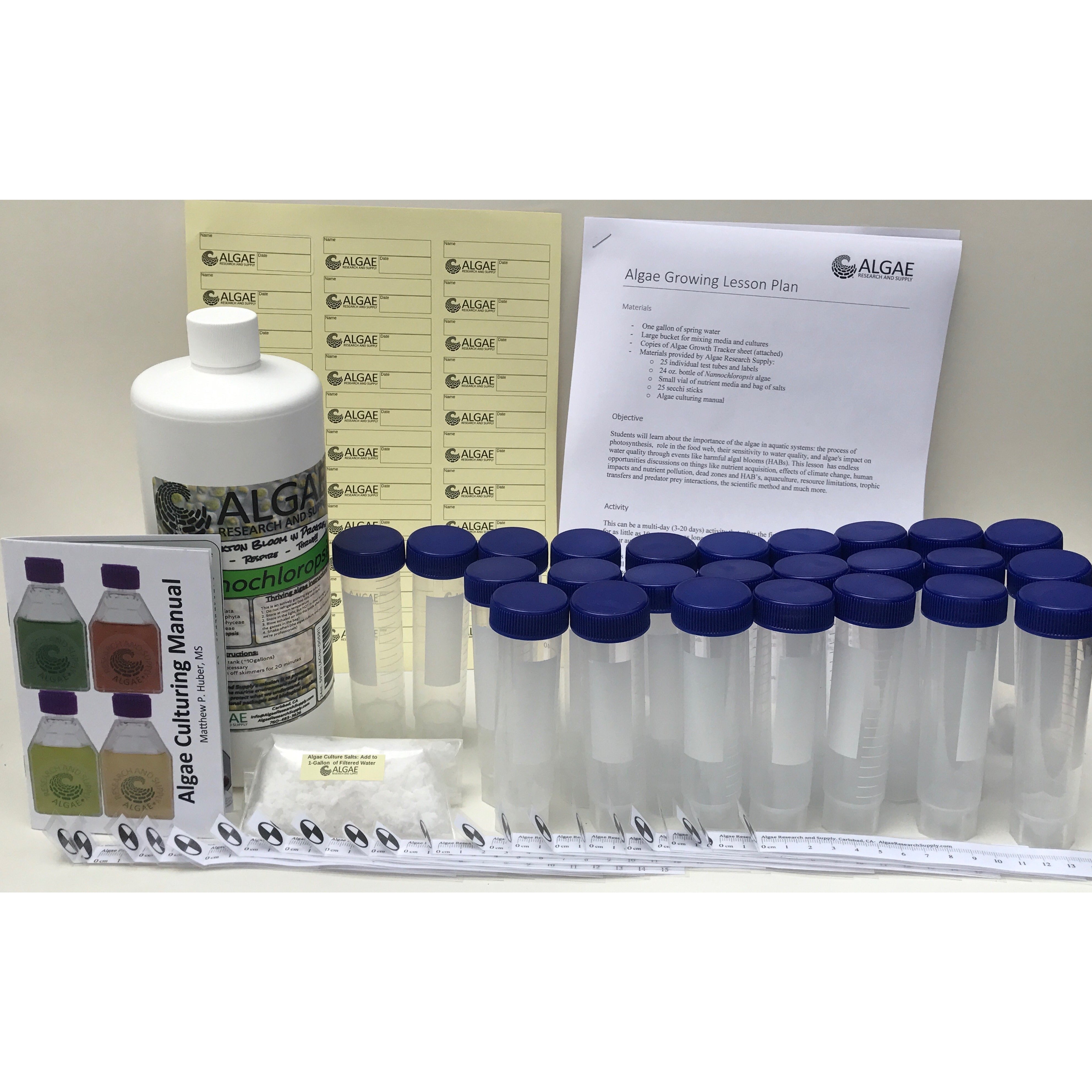
Ignite curiosity and foster hands-on learning with our comprehensive algae experiment kits. Designed for educators, students, and science enthusiasts, each kit provides all necessary materials to explore the fascinating world of algae. From culturing diverse algal species to conducting experiments on growth conditions, these kits offer a practical approach to understanding ecological and biological concepts. Ideal for classrooms, science fairs, or personal exploration, our kits make the study of algae accessible and engaging for all ages.